The extraction of compounds from solid samples is a fundamental technique in many scientific disciplines. In fields such as analytical chemistry, pharmacology, food science, biotechnology, and environmental analysis, having efficient methods to isolate active principles or contaminants is crucial for obtaining accurate and reproducible results.
Among the most recognized and widely used laboratory methods is Soxhlet extraction, a classical and highly effective solid-liquid extraction technique. Developed in 1879 by German chemist Franz von Soxhlet, this method revolutionized the way scientists extract target compounds from solid matrices. Its simplicity, reproducibility, and efficiency make it an indispensable tool, even in the age of modern extraction technologies.
This article explores the Soxhlet method in depth, breaking down the operation of the Soxhlet equipment, each of its components, and the cyclic process that defines it. We will also discuss its most common applications, benefits, limitations, and provide answers to frequently asked questions about using the Soxhlet extractor in the laboratory.
What Is the Soxhlet Method? Principles of Extraction
Soxhlet extraction is a solid-liquid extraction technique used to isolate soluble compounds contained in a solid sample using a suitable solvent. Unlike simple maceration or decoction methods, the Soxhlet technique enables continuous extraction using a fixed amount of solvent that is recycled multiple times through the sample.
The Soxhlet method is based on the principle of recycling heated solvent, which evaporates, condenses, and repeatedly passes through the solid sample contained in a cellulose thimble. As the fresh solvent comes into contact with the sample, it dissolves the target compounds. Once a certain volume is reached in the extraction chamber, the system automatically siphons the loaded solvent back into the flask, and the cycle repeats.
The main advantage of the Soxhlet method is that the sample is continuously in contact with fresh solvent, which maximizes extraction efficiency. Additionally, the system uses a limited amount of solvent since it is recycled.
General Steps of the Cycle
- Evaporation of the solvent from a heated flask.
- Condensation of the vapor in a condenser.
- Percolation of the solvent over the sample in the extractor.
- Automatic siphoning of the loaded solvent.
- Cycle restarts with solvent returning to the flask.
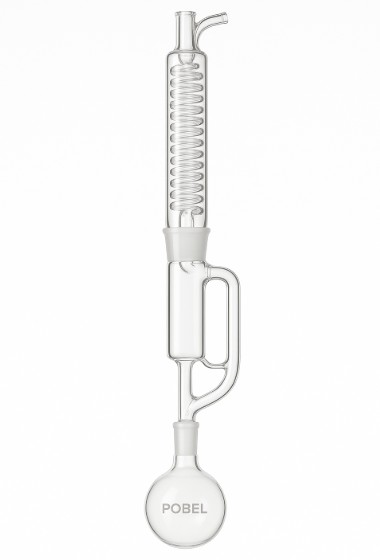
Components of the Soxhlet Equipment: An Ingenious System
The Soxhlet apparatus is a prime example of simple yet highly functional glass engineering. It consists of several components that together enable the cyclic extraction process. Below are the main components of the Soxhlet equipment:
Round-Bottom Flask
This is the container where the solvent is placed. It is connected to a heating source (such as a heating mantle or oil bath) to evaporate the solvent. It also collects the extract at the end of the process.
Soxhlet Extractor Body
This is the core of the system. It contains a central compartment where the solid sample is placed, usually inside a cellulose thimble. This body also includes a siphon tube that regulates the automatic draining of the solvent back into the flask.
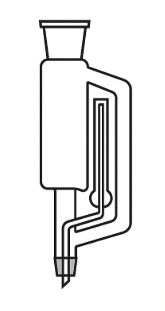
Condenser
Located at the top of the system, the condenser condenses the vaporized solvent coming from the flask. Typically, it’s a Liebig or Allihn condenser cooled by running water.
Heat Source
This can be a heating mantle or oil bath that keeps the solvent at a gentle boil. Consistent and controlled heating is essential.
Clamps and Supports
These are necessary to secure the equipment vertically and avoid accidents or spills in the lab.
How Does the Soxhlet Extractor Work? The Extraction Cycle
The Soxhlet extractor operates in a continuous and automated cycle thanks to its design. Below is a step-by-step explanation of how the entire extraction cycle works:
1. Evaporation
The solvent in the flask is heated and begins to evaporate. The vapor travels upward through the extractor's side tube.
2. Condensation
As the vapor reaches the condenser, it cools and turns back into liquid. This condensed solvent then drips onto the sample held in the thimble inside the Soxhlet extractor.
3. Immersion Extraction
The fresh solvent comes into contact with the sample, dissolving the soluble compounds. This process is more efficient than single-step extraction since fresh solvent is continuously used.
4. Siphoning
Once the solvent volume in the extractor chamber reaches the siphon tube’s height, it is automatically drained back into the flask, carrying the extracted compounds with it.
5. Recycling
The solvent is reheated, and the cycle repeats. The number of cycles depends on the target compound and the desired extraction yield.
Duration
A Soxhlet extraction may last anywhere from 3 to 24 hours, depending on the analyte, the sample type, the mass used, and the selected solvent.
What Is the Soxhlet Method Used For? Main Applications
Soxhlet extraction is widely used in academic, industrial, and research laboratories. Its main applications include:
Lipid and Fat Extraction
One of the most classical applications is determining fat content in foods, such as oilseeds, nuts, meats, or dairy products.
Isolation of Natural Compounds
In phytotherapy and plant biotechnology, it is used to extract alkaloids, flavonoids, terpenes, and other secondary metabolites from medicinal plants.
Pharmaceutical Analysis
In pharmacology, it allows for the extraction and quantification of active ingredients from raw plant or animal materials.
Environmental Analysis
Used to extract persistent organic pollutants such as pesticides or polycyclic aromatic hydrocarbons (PAHs) from soils and sediments.
Quality Control
Industries such as food, cosmetics, and petrochemicals use the Soxhlet method to validate ingredient concentrations or detect contaminants.
Materials
In materials science and polymer engineering, it helps remove unpolymerized residues, plasticizers, or other soluble additives from solid matrices.
Advantages and Limitations of Using Soxhlet Equipment
Advantages
The Soxhlet extraction technique offers a number of benefits that have established it as an essential tool in the laboratory, even more than a century after its invention. Below are its main advantages, explained in greater detail:
• High extraction efficiency due to continuous contact with fresh solvent
One of the most notable features of the Soxhlet method is its high efficiency in recovering target compounds. Thanks to the design of the extractor, the solvent in contact with the solid sample is always fresh and pure, having just been condensed. This optimizes the dissolution of analytes and ensures that a favorable concentration gradient is maintained throughout the process. As the cycles repeat, nearly complete extraction of the desired compounds is achieved—especially useful when quantitative recovery is the goal.
• Simple operation, requiring minimal manual intervention
Unlike other extraction methods that demand manual stirring, frequent filtering, or solvent replacement, the Soxhlet system operates almost autonomously once assembled. After starting the heating source, the system runs through automated cycles of evaporation, condensation, immersion, and siphoning. This frees up valuable time for the analyst, who can focus on other tasks while the equipment operates. Additionally, the setup requires no sophisticated instruments or specialized software, making it accessible even in low-resource laboratories.
• Reproducible results in quantitative analyses
Soxhlet extraction has been used for decades in official analytical methods—such as those established by the AOAC (Association of Official Analytical Collaboration)—which is a testament to its reliability. As a closed and automated system, it has fewer variables that could impact yield. This consistency in experimental conditions enhances the reproducibility of results, a key requirement in scientific research, product development, and quality control.
• Low solvent consumption compared to successive manual extractions
Although it may initially seem that continuous solvent use would be wasteful, Soxhlet extraction is actually quite efficient in this regard. Unlike manual techniques that require fresh solvent for each step, the Soxhlet system recirculates a fixed volume of solvent through numerous cycles. This significantly reduces the total volume needed for thorough extraction—resulting in cost savings and less chemical waste, contributing to greener laboratory practices.
• Versatility: applicable to a wide range of matrices and compounds
Soxhlet equipment can be adapted to extract compounds from a wide variety of solid samples, from plant tissues and food to soils, plastics, and synthetic materials. It also accommodates many types of solvents, allowing users to adjust for different polarities and target compounds. This flexibility makes the Soxhlet method suitable for multiple scientific disciplines: organic chemistry, food science, pharmacy, environmental toxicology, petrochemistry, and more.
Limitations
Despite its many strengths, the Soxhlet method does have some drawbacks, particularly when compared to more modern extraction techniques. Understanding these limitations is essential when selecting the best extraction strategy for a given analysis.
• Lengthy extraction time, especially compared to modern techniques like microwave- or ultrasound-assisted extraction
One of the main disadvantages of the Soxhlet method is its slow processing time. Depending on the type of sample and target compounds, the extraction may take anywhere from 4 to 24 hours. This is a stark contrast to emerging techniques like microwave-assisted or ultrasonic extraction, which can achieve comparable yields in just a few minutes. For settings where time is critical—such as high-throughput labs—this can be a significant limitation.
• Continuous heat exposure, which may degrade thermolabile compounds
Soxhlet extraction operates under constant heating conditions necessary for solvent evaporation. Although the solvents used often have relatively low boiling points, prolonged exposure to elevated temperatures can degrade heat-sensitive compounds such as vitamins, enzymes, flavonoids, or essential oils. In such cases, the chemical integrity of the analyte may be compromised, affecting both quantification and subsequent characterization.
• Not suitable for highly volatile or flammable solvents without specialized safety systems
Using volatile organic solvents such as ether, acetone, or chloroform involves inherent risks when working with open or semi-open systems like the Soxhlet extractor. Prolonged heating of these solvents can generate flammable vapors, requiring fume hoods and strict safety protocols. Although some labs use modified Soxhlet systems with inert atmospheres or pressurized setups, these are not standard and can represent a significant investment.
• Limited scalability: ideal for lab-scale use but not for industrial processing
The Soxhlet system is designed for small-scale volumes, making it ideal for research, exploratory analysis, and laboratory testing. However, its capacity is limited when larger quantities need to be processed for industrial or production purposes. In such cases, continuous or semi-continuous column extraction systems—often operated under pressure—are preferred, as they allow for faster and more efficient processing of large batches.
Frequently Asked Questions About Soxhlet Extraction
What type of solvent should I use in a Soxhlet extraction?
The solvent should be chosen based on the target compound’s polarity and solubility. Common solvents include hexane, ethanol, petroleum ether, dichloromethane, and methanol. It should have a moderate boiling point and high affinity for the analyte.
How long should a Soxhlet extraction take?
It depends on the sample and target compound, but typically between 4 and 8 hours. Some standardized analytical methods specify fixed durations to ensure reproducibility.
Can thermolabile compounds be extracted with Soxhlet?
It is not ideal since the process involves constant heat. In such cases, alternative methods like cold extraction, ultrasound-assisted, or supercritical fluid extraction are preferred.
What is the Soxhlet thimble or extraction cartridge?
It is a porous cellulose cylinder that holds the solid sample. Its purpose is to allow solvent flow while retaining solid particles, keeping the system clean.